Welcome to the Santala Research Group!
We use transmission electron microscopy, including aberration-corrected (S)TEM and photo-emission TEM, and other characterization techniques to study:
This research thrust focuses on the atomic-level structural characterization of metal/γ-alumina interfaces and is in collaboration with Dr. Líney Árnadóttír. Novel processing methods and aberration-correction electron microscopy are used for the experimental characterization of metal/γ-alumina interfaces. Density functional theory (DFT) based calculations are used to model and evaluate interfacial structures and to connect structural information to the thermodynamic properties in the interface, including the interfacial free energy. Ion implantation followed by thermal annealing is used to produce well-defined, model metal/γ-alumina interfaces with catalytically important metal species enabling atomic resolution imaging of both phases simultaneously. The project utilizes aberration-corrected TEM and scanning TEM (STEM) to produce high quality quantitative structural data regarding the relative position of all chemical species (Al, O, and the metal) that can be meaningfully compared to DFT-based calculations of the structure and thermodynamic stability ranges of interfaces with different γ-alumina chemical terminations.
This work has been supported by the National Science Foundation under Grant No. 1610507. Any stated opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Above: Platinum nanoparticles in Θ-alumina. Taken with the TEAM 0.5 at the National Center for Electron Microscopy
Kinetics and thermodynamics of crystallization of amorphous semiconductors and phase change materials
Crystallization kinetics are key to understanding glass stability across all classes of materials. The data needed to extract important relationships between viscosity, crystal growth rate, and temperature are incomplete for important materials that are marginal glass formers, such as phase change materials (PCMs). PCMs are (semiconducting) alloys used in optical- and resistivity-based memory owing to their fast switching between amorphous and crystalline states. The relationship between crystal growth and viscosity with changes in temperature is not fully understood because crystallization can be so rapid the measurement of physical and thermodynamic properties during crystallization is frustrated. Multiple recent reports of crystal growth and viscosity behavior extracted from calorimetric measurements do not satisfactorily fit existing models of crystal growth. This discrepancy may be due to changes in crystallization mechanism and flawed assumptions about the relative contribution of nucleation and growth in different temperature regimes. In this research thrust, crystallization is being studied with in situ microscopic methods, enabling direct observation of crystal growth and revealing changes in phase transformation mechanisms. Recent advances in transmission electron microscopy (TEM) techniques have made this endeavor possible even at temperatures where crystal growth is extremely rapid. In situ TEM with simultaneous nanocalorimetry will provide a seamless connection between thermodynamic and kinetic data. Knowledge resulting from this research advances the understanding of glass stability in PCMs and is of value to scientific communities researching materials for low-power, non-volatile memory and other marginal glass formers, such as bulk metallic glasses.
This work is being supported by the National Science Foundation under Grant No. 1945520. Any stated opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
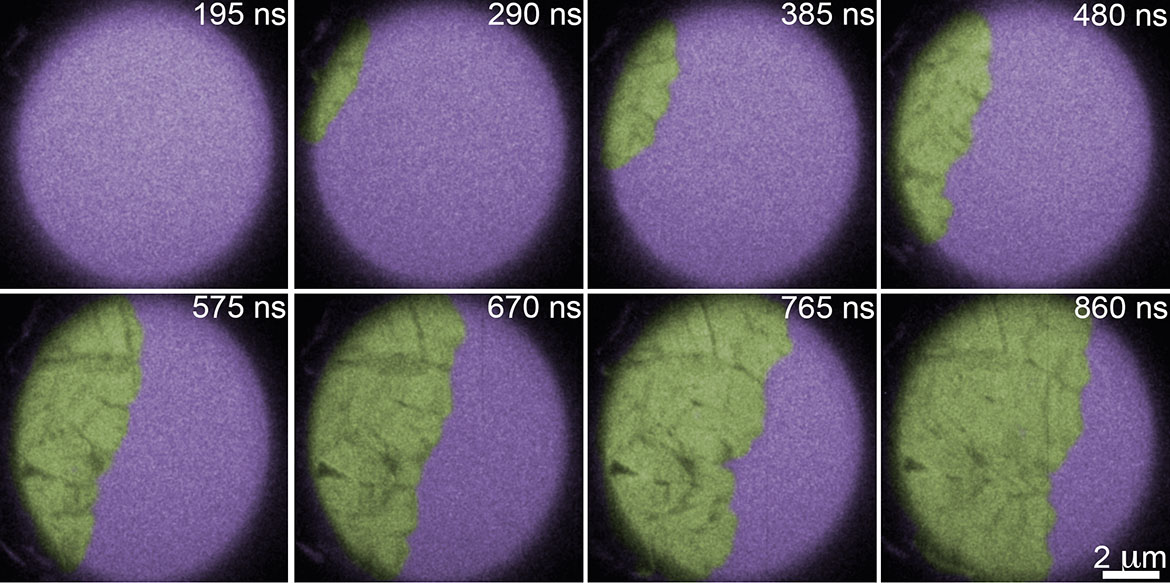
Above: False color image of amorphous Ge (purple) crystallizing after laser heating. The crystalline front progagates at approximately 10 m/s. Imaged with the Dynamic TEM at Lawrence Livermore National Laboratory.